Conditions d’achèvement
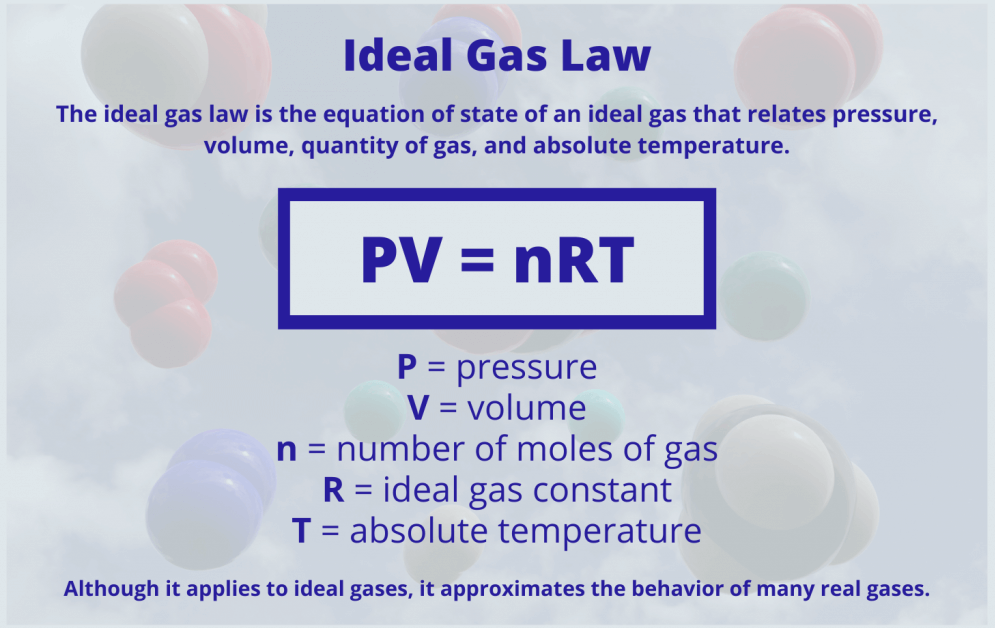

I.6 Perfect gas
This is a theoretical gas model in which, apart from collisions, no account is taken of interactions between molecules.
PV = n RT
P: pressure (atm, Pa, 1 atm = 1.013 105 Pa, mmHg)
V : volume (L, m3 1L = 10-3 m3)
N: number of moles (mol)
R: perfect gas constant (R = 0.0082 L am/mol K = 8.31 J /mol K = 2 cal/mol K)
T : temperature (°C, K, T (K) = T (°C) + 273)
