Conditions d’achèvement
IV. Notions of atoms and molecules
IV. Notions of atoms and molecules
IV.1. Structure of the atom
IV.1.1. The nucleus and electrons
Atoms are made up of a very dense, positively charged nucleus surrounded by a cloud of negatively charged electrons. The nucleus is made up of two types of particles (protons and neutrons) called nucleons.
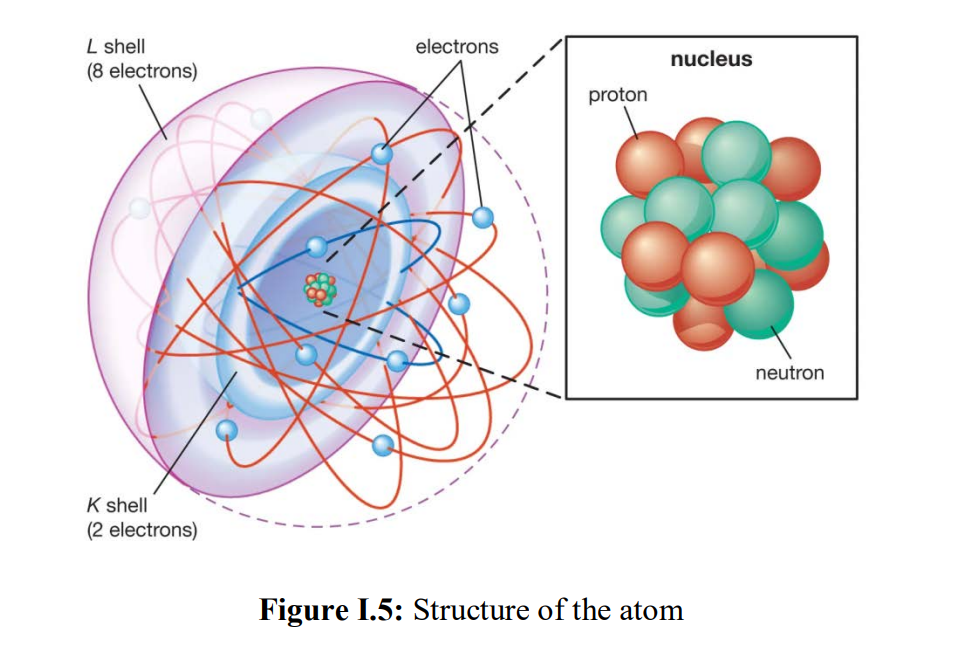
Shell atomic model in the shell atomic model, electrons occupy different energy levels, or shells. The K and L shells are shown for a neon atom.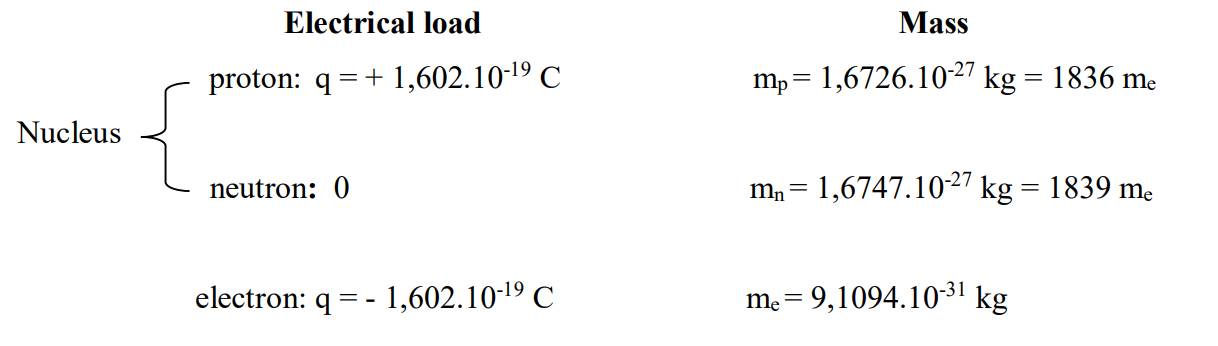
IV.1.2. The nuclide
A nuclide is an atomic species symbolized by: Isotope Symbol
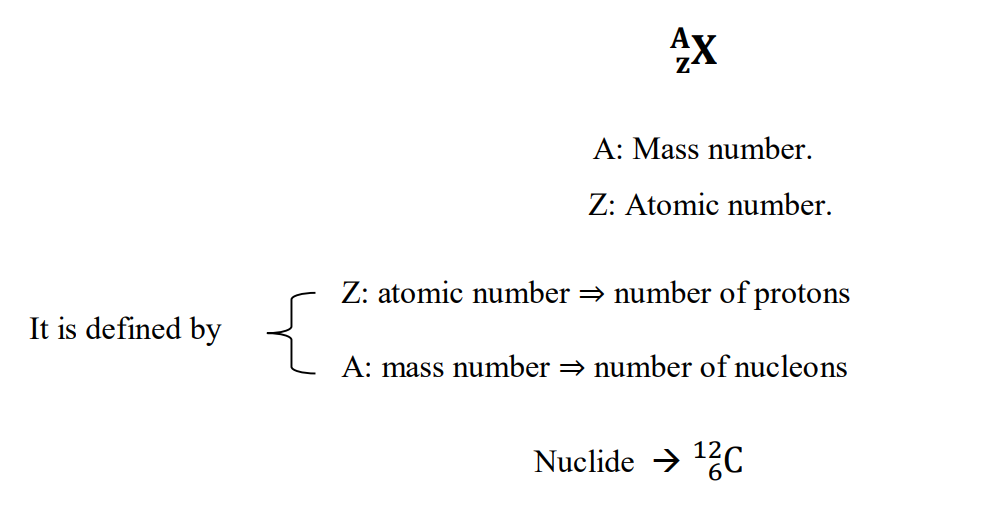
IV.1.3. Element isotopes
A = Z + N
Hence the number of neutrons:
N = A – Z Nuclides with the same number of protons (same Z) correspond to the same element.
They have the same name. Example: Magnesium:
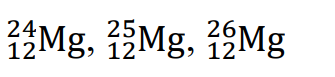 Isotopes of an element are nuclides with the same atomic number Z, but different mass numbers
A.
Isotopes of an element are nuclides with the same atomic number Z, but different mass numbers
A.
Example:
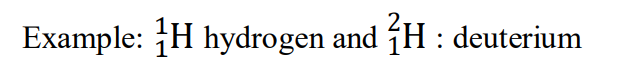 : deuterium
- Real atomic mass = mass of a real atom: expressed in kg or in u.m.a. (u) (atomic mass unit). The
isotope is used as a reference: it is assumed that a real atom weighing 1,99625.10-26 kg corresponds
to exactly 12 u ⇒ 1 u = 1,66054.10-27 kg
⇒ 1 u ≈ mp ≈ mn
: deuterium
- Real atomic mass = mass of a real atom: expressed in kg or in u.m.a. (u) (atomic mass unit). The
isotope is used as a reference: it is assumed that a real atom weighing 1,99625.10-26 kg corresponds
to exactly 12 u ⇒ 1 u = 1,66054.10-27 kg
⇒ 1 u ≈ mp ≈ mn
IV.1.4. Avogadro number NA
The number of real atoms contained in 1 mole of the isotope
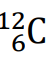 of carbon.
of carbon.