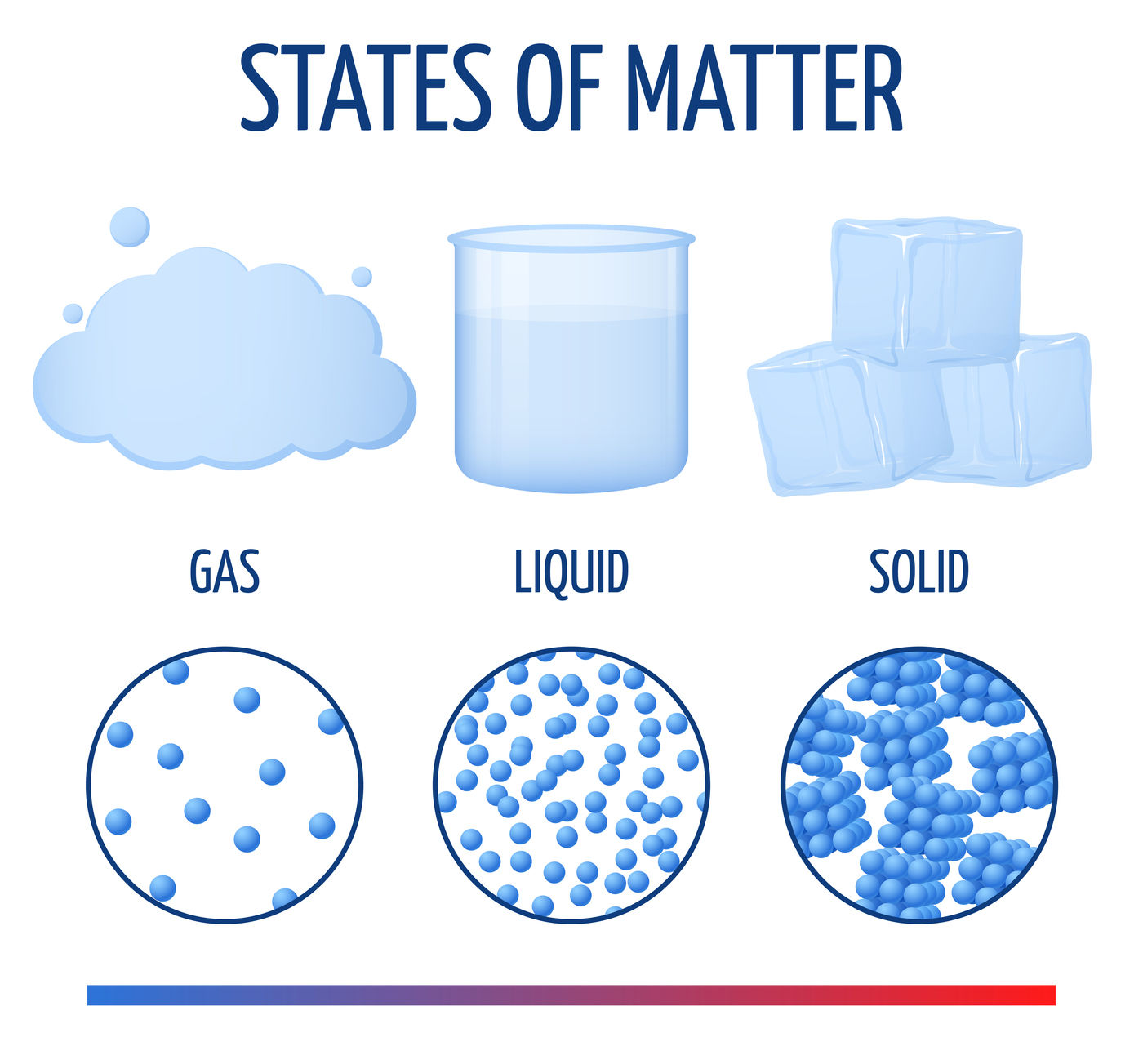
I. The state of matter What is Matter in Chemistry?
the field of chemistry, matter refers to any substance that occupies space and possesses mass. According to scientific findings, matter consists of little particles that are imperceptible to the naked sight. It has been empirically noted that matter manifests in several states throughout the natural world. Certain substances exhibit rigidity and maintain a stable shape, such as wood and stone. Conversely, other substances possess the ability to flow and conform to the shape of their container, as exemplified by water. Additionally, there exist forms of matter, such as air, that lack a definitive shape or size. Matter can be categorized into distinct classifications based on their observable physical qualities and the various states in which they manifest. These classifications are commonly referred to as states of matter. There are three states of matter: solid, liquid, and gas.
I.1. Matter Definition Chemistry
Chemistry is an academic discipline that focuses on the investigation of the constituent elements and their respective arrangements within matter, as well as the examination of the many processes by which matter undergoes alteration or conversion. The term "substance" is frequently seen as interchangeable with "matter"; but, within the realm of chemistry, a substance is defined more restrictively. Chemistry encompasses the investigation of the properties and interactions of substances. Chemistry encompasses the study of the composition, structure, and properties of matter, as well as the investigation of the phenomena that arise when various types of matter undergo transformations. The concept of matter theory encompasses the evolving concepts and frameworks employed to elucidate and comprehend the physical realm. A significant portion of matter theory was founded upon a theoretical framework concerning the fundamental constituents known as the elements.
I.2. Solid Definition
• In solids, particles are tightly or closely packed.
• The gaps between the particles are tiny and hence it is tough to compress them.
• Solid has a fixed shape and volume.
• Due to its rigid nature, particles in solid can only vibrate about their mean position and cannot move.
• Force of attraction between particles is adamant.
• The rate of diffusion in solids is very low.
• An example of solids: solid ice, sugar, rock, wood, etc.
I.3. Liquid Definition
• In a liquid state of matter, particles are less tightly packed as compared to solids.
• Liquids take the shape of the container in which they are kept.
• Liquids are difficult to compress as particles have less space between them to move.
• Liquids have fixed volume but no fixed shape.
• The rate of diffusion in liquids is higher than that of solids.
• Force of attraction between the particles is weaker than solids.
• Example of a liquid state of matter: water, milk, blood, coffee, etc
I.4. Gas Definition
• In gases, particles are far apart from each other.
• Force of attraction between the particles is negligible, and they can move freely.
• Gases have neither a fixed volume nor a fixed shape.
• The gaseous state has the highest compressibility as compared to solids and liquids.
• The rate is diffusion is higher than solids and liquids.
• The kinetic energy of particles is higher than in solids and liquids.
• An example of gases: air, helium, nitrogen, oxygen, carbon dioxide, etc.