Conditions d’achèvement
Support resources.

We provide you with resources (located on
a digital platform) that will help you understand the Structure of Matter
module course. All programmed courses are available in PDF and SCORM format,
with examples and applications.
1.
In addition to the following references:
2. BibliographyChimie générale – Exercices et Méthodes, 2015, Licence
– PACES – CAPES Sous la direction de Danielle Baeyens-Volant, Nathalie Warz
3. Maxi fiches de Chimie générale.2011. Dunod Yann Verchier,
Anne-Laure Valette. Delahaye, Frédéric Lemaître
4. Chimie générale - Exercices et problèmes, Rappels de cours,
exercices avec corrigés détaillés Licence, PCEM 1, PH1,Élisabeth Bardez 2009
Sciences Sup.
5. Chimie générale – Tout le cours en fiches – 2e éd., 2016, Licence
– PACES – CAPES Sous la direction d'Alain Sevin.
6. https://ptable.com/?lang=fr#Orbital
7. http://www.sciences-en[1]ligne.com/DIST/Data/Ressources/lic2/chimie/chi_gen/Chi_gen_sommaire.htm
8. https://moodle.umontpellier.fr/course/view.php?id=575
9.
Chemguide.co.uk, (2015). atomic orbitals.
Retrieved 28 September 2015, from http://www.chemguide.co.uk/atoms/properties/atomorbs.html
10. Chemwiki.ucdavis.edu,
(2015). Atomic orbitals. Retrieved 27 September 2015, from 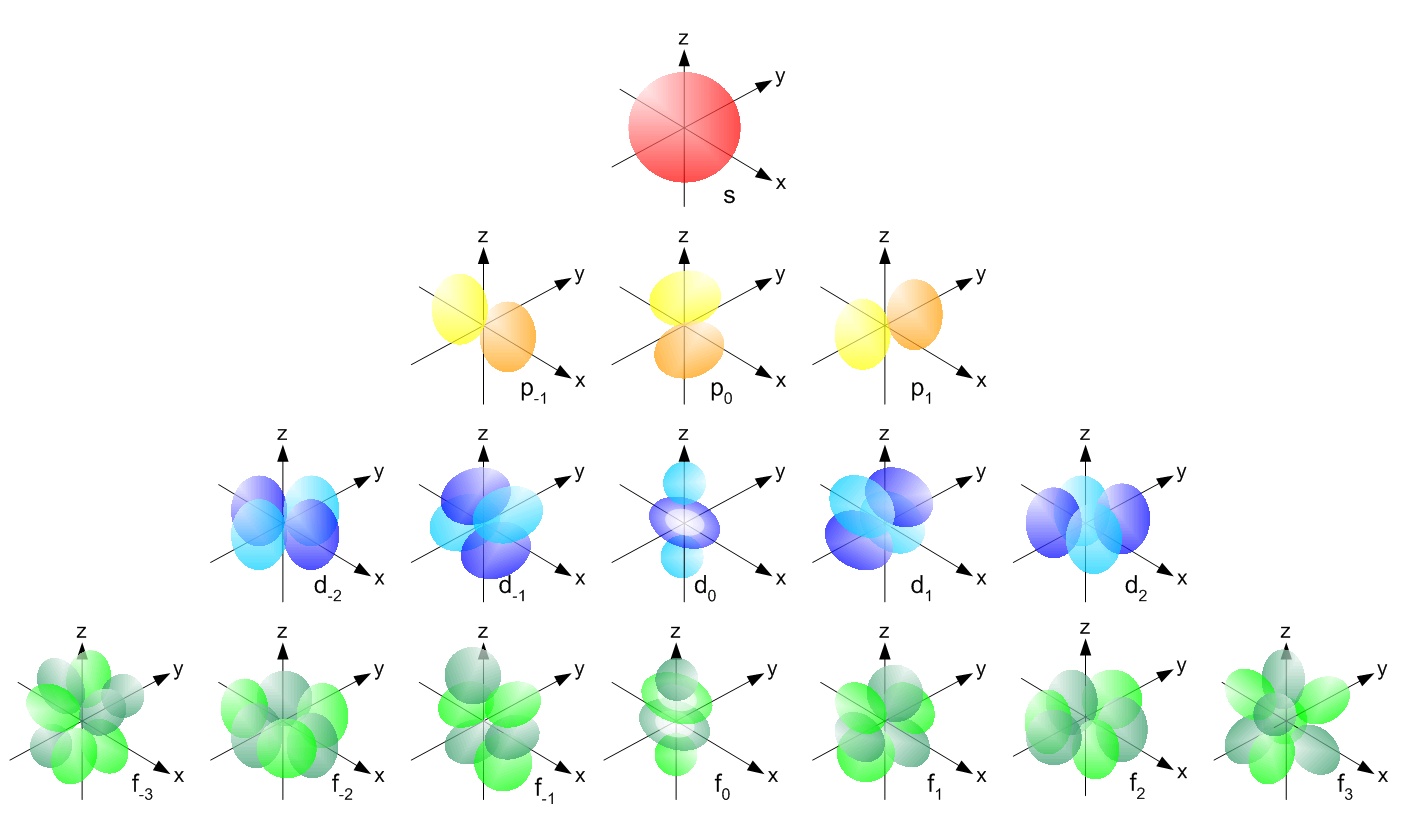
11. Chemwiki.ucdavis.edu,
(2014). 6.9: Electron Configurations and the Periodic Table -Chemwiki.
Retrieved 2 October 2015, from http://chemwiki.ucdavis.edu/?title=Textbook
12. Maps/General
Chemistry Textbook Maps/Map: Brown, LeMay, %26 Bursten %22Chemistry:
13. The
Central Science%22/06. Electronic Structure of Atoms/6.9: Electron
Configurations and the Periodic Table
14. Jha,
A. (2013), What is Heisenberg's Uncertainty Principle? The Guardian. Retrieved
27
15. September
2015, from http://www.theguardian.com/science/2013/nov/10/what-is[1]heisenbergs-uncertainty-principle
16. Mpcfaculty.net,
(2015). Complete Electron Configurations. Retrieved 30 September 2015, from
http://www.mpcfaculty.net/mark_bishop/complete_electron_configuration_help.htm
17. Nano.gov.
(2015), what is Nanotechnology? | Nano. Retrieved 5 November 2015, from http://www.nano.gov/nanotech-101/what/definition
18. Wwu.edu,
(2015). Stardust. Retrieved 27 September 2015, from http://www.wwu.edu/skywise/a101_dust.html
19. Atkins,
Peter and Julio de Paula. Physical Chemistry for the Life Sciences. New York: Oxford University Press, 2006.
20. Chang,
Raymond. Physical Chemistry for the Biosciences. USA:
University Science Books, 2005.
21. Gore,
Michael. Spectrophotometry & Spectrofluorimetry. New York: Oxford
University Press, 2000.
22. Price,
Nicholas and Dwek, Raymond and Wormald, Mark. Principles and Problems in
Physical
23. Chemistry
for Biochemists. R. G. Ratcliffe. New York: Oxford University Press,1997.
24. •
Irwin H. Segel, Biochemical Calculations (How to Solve Mathematical Problems in
General
25. Biochemistry),
2nd edition, John Wiley & Sons, 1975
26. http://www.nist.gov/pml/div685/grp03/spectrophotometry.cfm.
