Conditions d’achèvement
Intensive and Extensive Properties of Matter - Chemistry
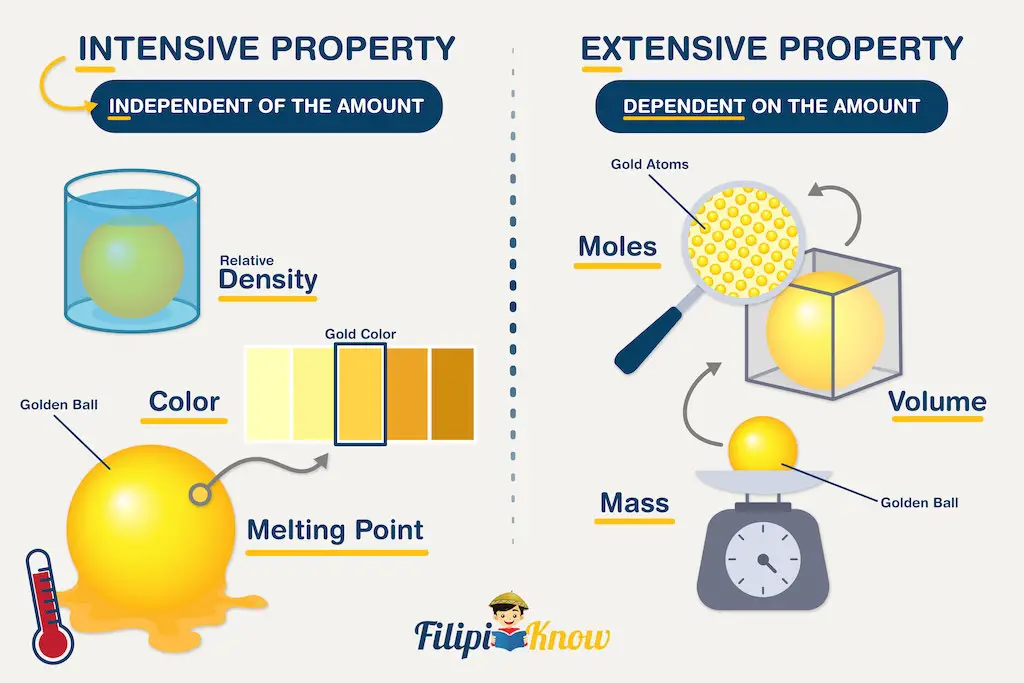
I.5.1 Intensive quantities (intensive variables)
These are independent of the quantity of matter in the system. These variables are non-additive.
Example
Pressure, temperature, mole fraction, etc.
I.5.2 Extensive quantities (extensive variables)
These are proportional to the quantity of matter in the system. They are additive variables.
Examples
Mass, volume, etc.
gas (m, v, T) + gas (m, v, T) gives→ gas (2m, 2v, T)
