Topic outline
-
👌Table of contents
I. Course information.
II. Course presentation.
III. Contents.
IV. Prerequisites.
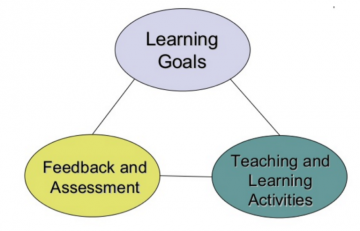
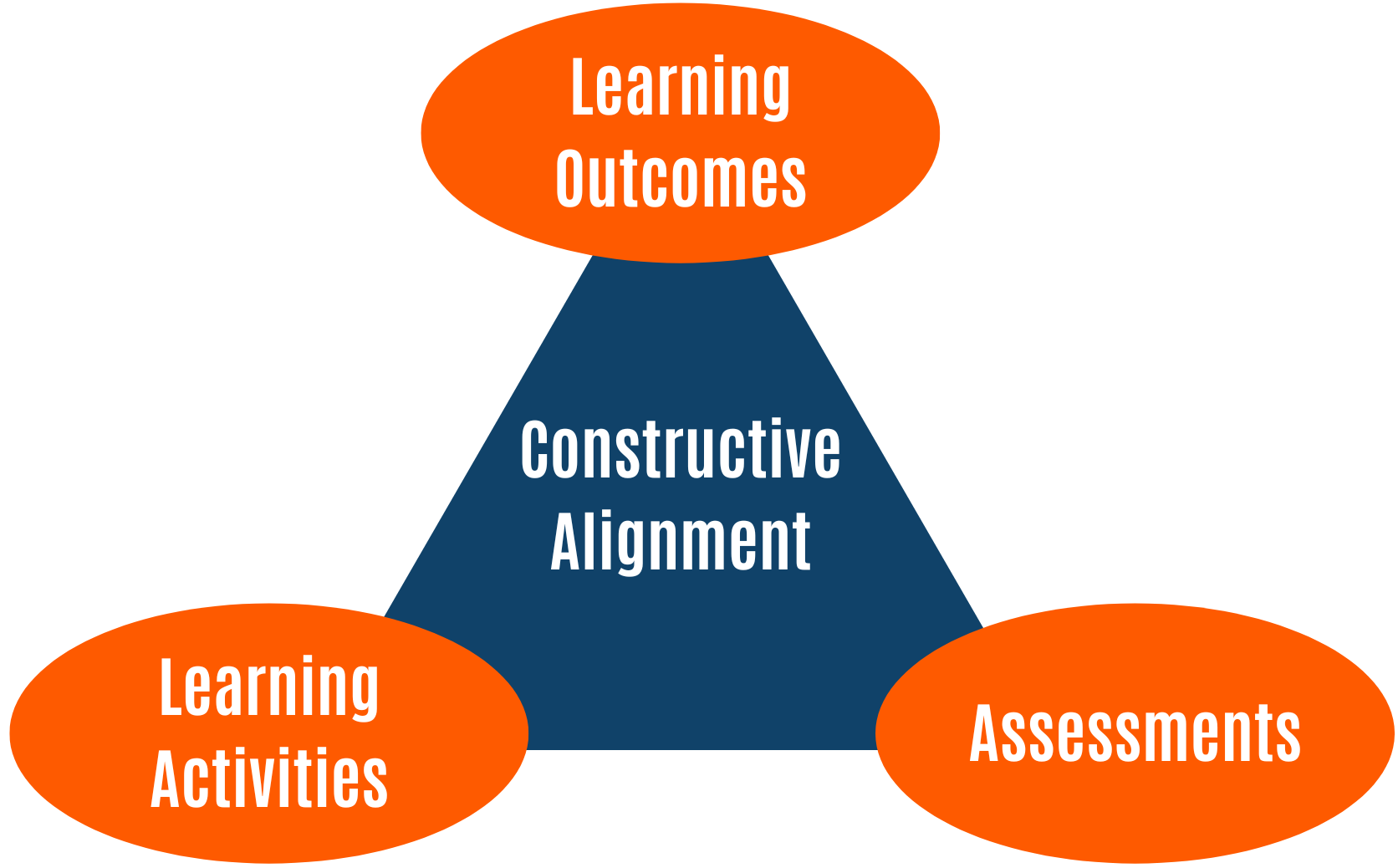
VI. Learning Evaluation.
VII. Teaching-learning activities.
IX. Operating procedures.

-
-
-

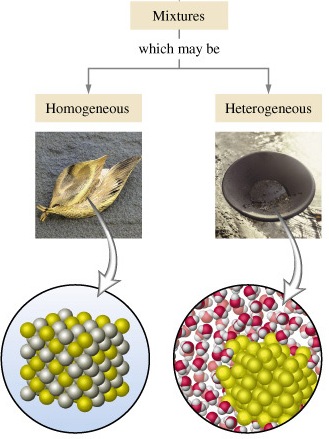
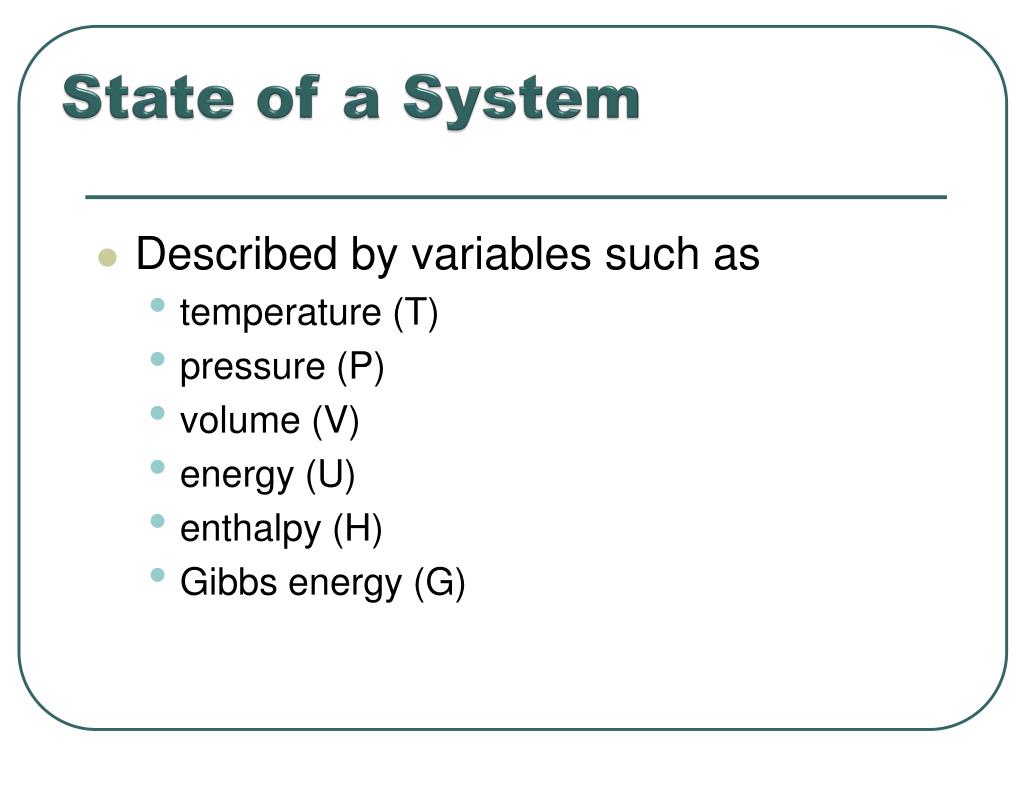
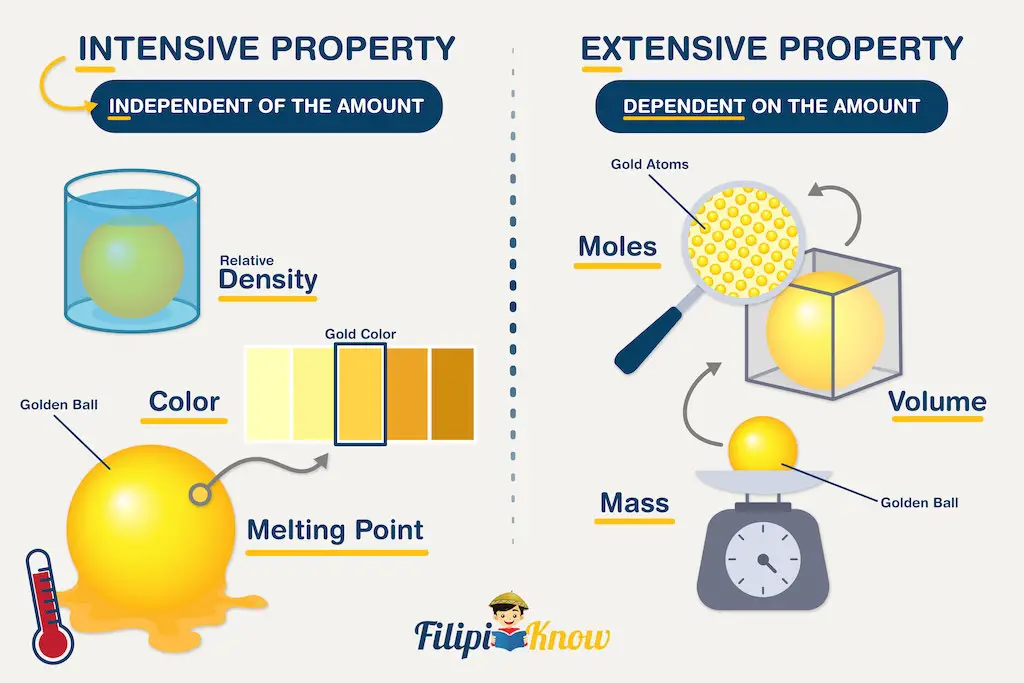
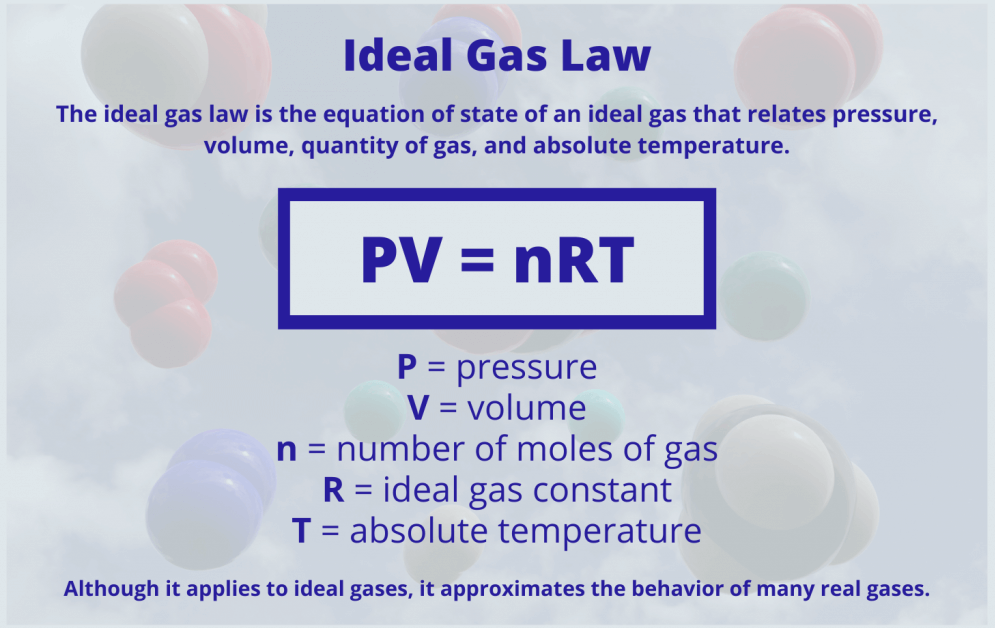
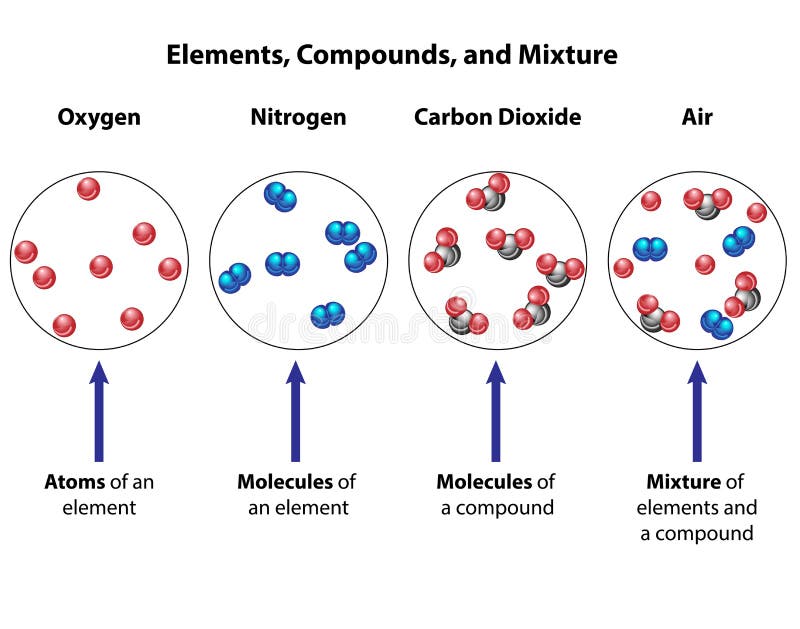
Like all sciences, thermodynamics is based on principles and laws formulated using a precise vocabulary. This allows concepts and definitions to be definitions without ambiguity. Chapter I therefore presents the basic elements study thermodynamics and solve practical problems. We begin the chapter with a general discussion of thermodynamics. The system of units used in this book is also clarified. Next, we define basic concepts system, control volume, thermodynamic variables, thermodynamic equilibrium, evolutions and cycles. The notions of temperature and temperature scales are introduced, then we determine the composition of gas mixtures.
-
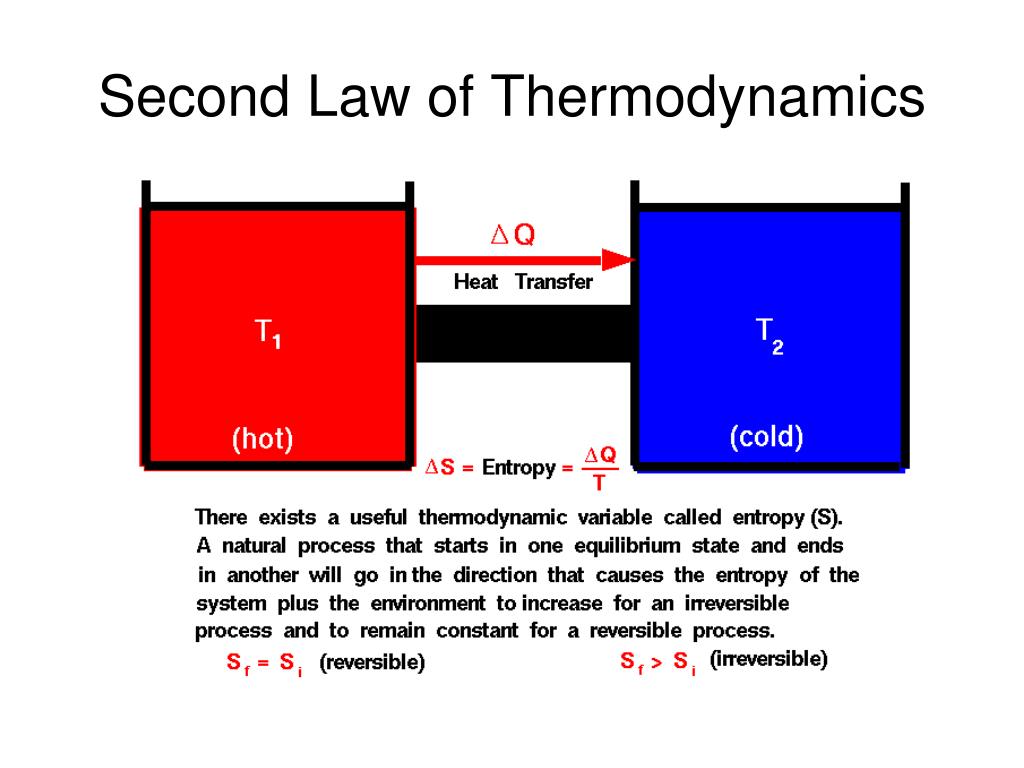
Reversible and Irreversible Thermodynamic Processes
The reversible process is the ideal process which never occurs, while the irreversible process is the natural process that is commonly found in nature. When we tear a page from our notebooks, we cannot change this and ‘un-tear’. This is an irreversible process. Whereas when water evaporates, it can also be condensed in the form of rains. This is a reversible process. Let us study more about them below.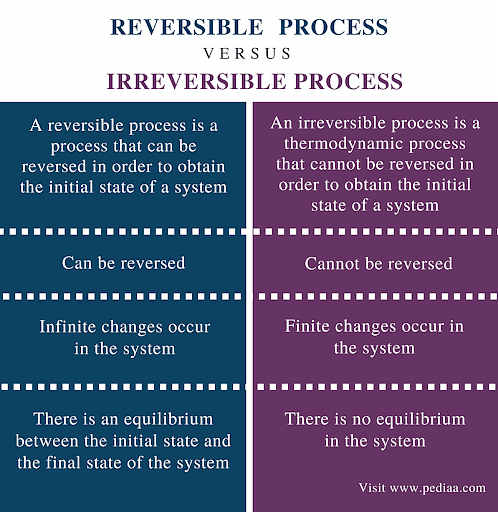
-



![A through Z of IT Standard Operating Procedures [Template] - Cimatri](https://cimatri.com/wp-content/uploads/2022/02/Standard-Operating-Procedures-Feature-Image.png)
![english1210asher [licensed for non-commercial use only] / Day 17](https://userscontent2.emaze.com/images/9c5c8ef6-9b6b-4db9-a8c4-6cde1928e98d/7ef886cf076ae6ea01544479f8560503.jpg)
![Thermodynamics System and It’s Types [With PDF] – Learn Mechanical](https://learnmechanical.com/wp-content/uploads/2019/07/THERMODYNAMIC-SYSTEMS.jpg)