Completion requirements
Reversible and Irreversible Thermodynamic Processes
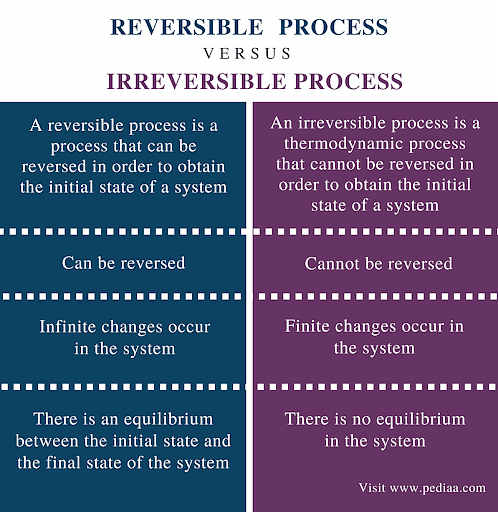
The reversible process is the ideal process which never occurs, while the irreversible process is the natural process that is commonly found in nature. When we tear a page from our notebooks, we cannot change this and ‘un-tear’. This is an irreversible process. Whereas when water evaporates, it can also be condensed in the form of rains. This is a reversible process. Let us study more about them below.
The reversible process is the ideal process which never occurs, while the irreversible process is the natural process that is commonly found in nature. When we tear a page from our notebooks, we cannot change this and ‘un-tear’. This is an irreversible process. Whereas when water evaporates, it can also be condensed in the form of rains. This is a reversible process. Let us study more about them below.
I.7 Transformation of a system
The transformation of a system is the passage of the system from a state of thermodynamic equilibrium, called the initial state, to another state of thermodynamic equilibrium, known as the final state.

